Radium
88
Ra
Gruppe
2
Periode
7
Blokk
s
Proton
Elektron
Nøytron
88
88
138
Generelle eigenskapar
Atomnummer
88
Atommasse
[226]
Massetal
226
Kategori
Jordalkalimetall
Farge
Sølv
Radioaktiv
Ja
From the Latin word radius meaning ray
Krystallstruktur
Romsentrert kubisk
Historie
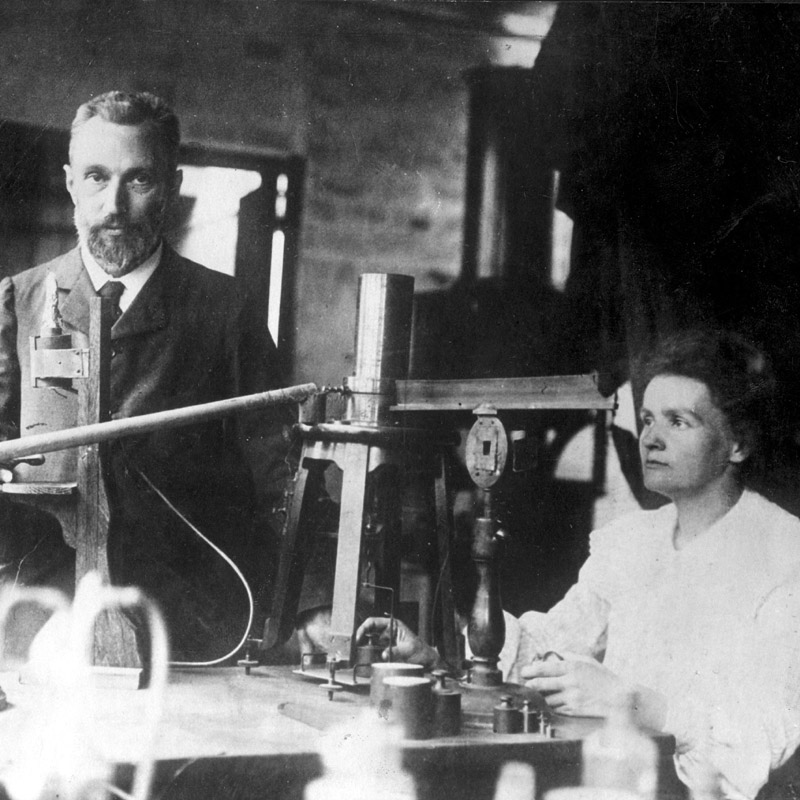
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektron per energinivå
2, 8, 18, 32, 18, 8, 2
Elektronkonfigurasjon
[Rn] 7s2
Radium imparts a carmine red color to a flame
Fysiske eigenskapar
Tilstandsform
Fast stoff
Tettleik
5,5 g/cm3
Smeltepunkt
973,15 K | 700 °C | 1292 °F
Kokepunkt
2010,15 K | 1737 °C | 3158,6 °F
Smeltevarme
8 kJ/mol
Fordampingsvarme
125 kJ/mol
Spesifikk varmekapasitet
- J/g·K
Førekomst i jordskorpa
9,9×10-12%
Førekomst i universet
i/t
CAS-nummer
7440-14-4
PubChem CID-nummer
6328144
Atom eigenskapar
Atomradius
-
Kovalent radius
221 pm
Elektronegativitet
0,9 (Paulings skala)
Ioniseringspotensial
5,2784 eV
Molart volum
45,20 cm3/mol
Termisk konduktivitet
0,186 W/cm·K
Oksidasjonstrinn
2
Bruksområde
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Isotopar
Stabile isotopar
-Ustabile isotopar
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra