Polonium
84
Po
Gruppe
16
Periode
6
Blokk
p
Proton
Elektron
Nøytron
84
84
126
Generelle eigenskapar
Atomnummer
84
Atommasse
[210]
Massetal
210
Kategori
Halvmetall
Farge
Sølv
Radioaktiv
Ja
Named after Poland, native country of Madam Curie
Krystallstruktur
Enkel kubisk
Historie
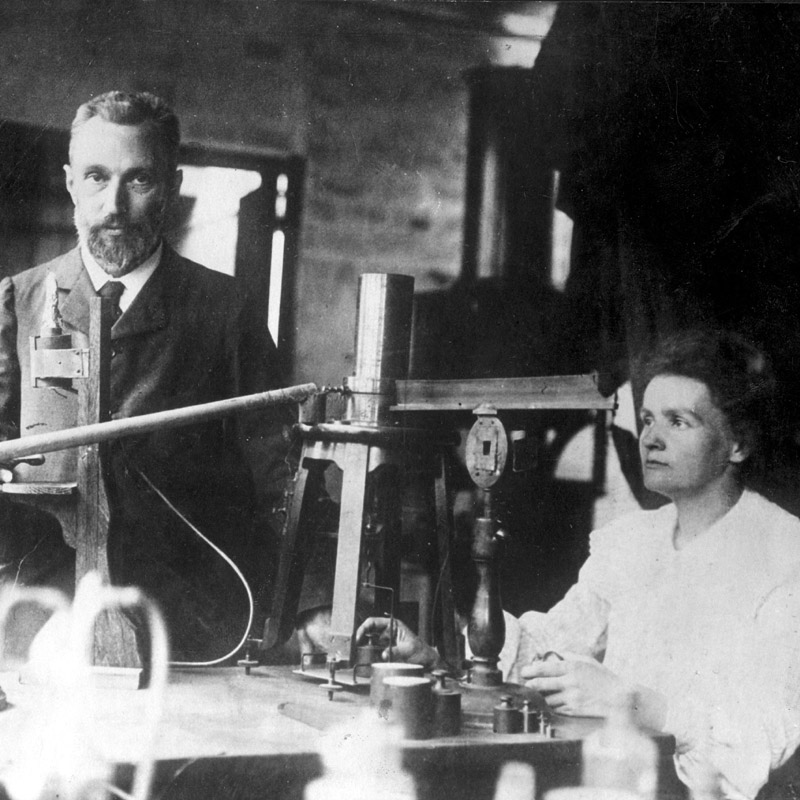
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Elektron per energinivå
2, 8, 18, 32, 18, 6
Elektronkonfigurasjon
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Fysiske eigenskapar
Tilstandsform
Fast stoff
Tettleik
9,196 g/cm3
Smeltepunkt
527,15 K | 254 °C | 489,2 °F
Kokepunkt
1235,15 K | 962 °C | 1763,6 °F
Smeltevarme
13 kJ/mol
Fordampingsvarme
100 kJ/mol
Spesifikk varmekapasitet
- J/g·K
Førekomst i jordskorpa
i/t
Førekomst i universet
i/t
CAS-nummer
7440-08-6
PubChem CID-nummer
i/t
Atom eigenskapar
Atomradius
168 pm
Kovalent radius
140 pm
Elektronegativitet
2,00 (Paulings skala)
Ioniseringspotensial
8,417 eV
Molart volum
22,23 cm3/mol
Termisk konduktivitet
0,2 W/cm·K
Oksidasjonstrinn
-2, 2, 4, 6
Bruksområde
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Isotopar
Stabile isotopar
-Ustabile isotopar
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po